 |
||
|
Impacts of warmer winters on the biological life |
||
|
1. Phytoplankton Warmer winters cause an earlier breakup of lake ice. As a consequence, an earlier occurrence of the spring phytoplankton bloom in the lakes Vänern, Vättern and Mälaren by about one month in the 1990's compared to the 1970's and 1980's has been observed1. This observation results from the fact that phytoplankton usually starts to bloom as soon as the lake ice disappears due to the increased availability of underwater light. Comparing ice free years in the lakes Vänern and Vättern with years with ice it was especially the diatom species Aulacoseira that increased2. Warmer winters also cause that the period when lake ice is covered by snow becomes shorter since snowfall is more and more replaced by rainfall. As a consequence, there are more occasions when light is able to pentrate through the snowfree ice cover, allowing a growth of dinoflagellates, an algal group that is able to develop under low light conditions. Since only few phytoplankton data below an ice cover are available from Sweden's largest lakes we can only assume that the lakes Vänern, Vättern and Mälaren behave similar to the relatively large Swedish Lake Erken where especially the specie Peridinium aciculiferum Lemmermann has been favoroured by less snow on the ice3. In combination with warmer winters also spring water temperatures increased in the lakes Vänern, Vättern and Mälaren1. This increase was probably the reason why blue-green algae in all three lakes occurred earlier (already in May) in the water column after warm winters1,4. Even if the biomass of the blue-green algae was still little in spring, the tendency of an earlier occurrence is undesirable since blue-green algae can become toxic when they occur in large amounts. No other phytoplankton group than blue-green algae showed a synchronous response to warmer winter temperatures among the 10 lake sites in the lakes Vänern, Vättern and Mälaren1. In Lake Mälaren it was observed that warmer water temperatures in combination with an increase in the water color led to an increase in the spring biomass of large Cryptophyceae5 (see figure). An increase in the biomass of Cryptophyceae might increase the smell of the water that is used as drinking water. 2. Zooplankton Zooplankton that uses phytoplankton as a food resource, usually shows a biomass peak short time after the phytoplankton spring bloom. Since the phytoplankton spring bloom shifted spring-ward as a result of warmer winters, we also observe a shift in the zooplankton biomass peak, occuring earlier after warm winters. This is reflected by a higher zooplankton biomass in May after warm winters in the lakes Vänern, Vättern and Mälaren1. 3. Fish The response of fish to warmer winters is complex and yet not completely analysed for Sweden's largest lakes Vänern, Vättern and Mälaren. One study shows that autumn spawners such as vendance (Coregonus albula) have difficulties in adapting to warmer temperatures. The catch of vendance drastically decreased when lake ice breakup was very early in the year6. Vendance 4. Benthic fauna In Lake Vänern, the population density of the glacial relict amphipod Monoporeia affinis (Lindström) was related to the spring maximum diatom biovolume7. It is therefore likely that changes in the spring phytoplankton bloom as a response to warmer winters cause also changes in the population density of the glacial relict amphipod Monoporeia affinis (Lindström). In Lake Vättern, no relationship was found, probably due to the larger depth and the lower nutrient content of the lake. Also in Lake Mälaren no relationship was found, but instead summer hypoxia (< 4 mg O2 L-1) seems to be an important regulating factor for the population density of Monoporeia affinis (Lindström)7. Since hypoxia is caused by increasing water temperatures it is likely that the population density of Monoporeia affinis (Lindström) also in this lake is affected by the changing climate. Monoporeia affinis (Lindström)
5. Macrophytes, benthic algae, bacterioplankton Due to the lack of data, no in-depth analyses have yet been carried out where changes in macrophytes, benthic algae and bacterio-plankton have been coupled to warmer winter temperatures. |
The diatom species Aulacoseira Blue-green algal bloom in Lake Mńlaren A common blue-green algal species that can become toxic: Aphanizomenon sp. 1 Weyhenmeyer, G. A. 2001. Warmer winters - are planktonic algal populations in Sweden's largest lakes affected? Ambio 30: 565-571. 2 Westöo, A.-K. 2004. Warmer winters - how are water chemistry and phytoplankton in Sweden's largest lakes affected (in Swedish)? Master Thesis. Department of Environmental Sciences, Swedish University of Agricultural Sciences. 3 Weyhenmeyer, G. A., T. Blenckner and K. Pettersson. 1999. Changes of the plankton spring outburst related to the North Atlantic Oscillation. Limnol. Oceanogr. 44: 1788-1792. 4 Weyhenmeyer, G. A., R. Adrian, U. Gaedke, D. M. Livingstone, S. C. Maberly. 2002. Response of phytoplankton in European lakes to a change in the North Atlantic Oscillation. Verh. Intern. Verein. Limnol. 28: 1436-1439. 5 Weyhenmeyer, G. A., Willén, E. and Sonesten, L. Effects of an extreme precipitation event: brownish lake water and an increase in the biomass of Cryptophyceae (manuscript). 6 Nyberg, P., Bergstrand, E., Degerman, E. and Enderlein, O. 2001. Recruitment of pelagic fish in an unstable climate: studies in Sweden's four largest lakes. Ambio 30: 559-564. 7 Goedkoop, W. and Johnson R. K. 2001. Factors affecting population fluctuations of the glacial relict amphipod Monoporeia affinis (Lindström) in Sweden's largest lakes. Ambio 30: 552-558. Further reading Adrian, R. and Deneke, R. 1996. Possible impact of mild winters on zooplankton succession in eutrophic lakes of the Atlantic European area. Freshwater Biology 36: 757-770. Adrian, R., Hintze, T., Walz, N., Hoeg, S. and Rusche, R. 1999. Effects of winter conditions on the plankton succession during spring in a shallow polymictic lake. Freshwater Biology. 41: 621-632. Anderson, N. T. 2000. Diatoms, temperature and climatic change. European Journal of Phycology 35: 307-314. Anneville, O., Souissi, S., Ibanez, F., Ginot, V., Druart, J. C., Angeli, N. 2002. Temporal mapping of phytoplankton assemblages in Lake Geneva: Annual and interannual changes in their patterns of succession. Limnol. Oceanogr. 47: 1355-1366. Arvola, L., Palomäki, A., Lehtinen, S. and Järvinen, M.. 2002. Phytoplankton community structure and biomass in two basins of a boreal lake in relation to local weather conditions and the North Atlantic Oscillation. Verh. Internat. Verein. Limnol. 28: 700-704. Belgrano, A., Lindahl, O. and Hernroth, B. 1999. North Atlantic Oscillation, primary productivity and toxic phytoplankton in the Gullmar Fjord, Sweden (1985-1996). Proc. R. Soc. Lond. B 266: 425-430. Blenckner, T. 2001. Climate related impacts on a lake - from physics to biology. PhD-thesis, Acta Universitatis Upsaliensis 674. Elliott J. M., Hurley M. A. and Maberly S. C. 2000. The emergence period of sea trout fry in a Lake District stream correlates with the North Atlantic Oscillation. Journal of Fish Biology, 56, 208-210. George, D. G. 2000. The impact of regional-scale changes in the weather on the long-term dynamics of Eudiaptomus and Daphnia in Esthwaite Water, Cumbria. Freshwater Biology 45: 111-121. Gerten, D. and Adrian, R. 2000. Climate-driven changes in spring plankton dynamics and the sensitivity of shallow polymictic lakes to the North Atlantic Oscillation. Limnol. Oceanogr. 45: 1058-1066. Gilbert, J. J. 1996. Effect of temperature on the response of planktonic rotifers to a toxic cyanobacterium. Ecology 77: 1174-1180. Ottersen, G., Planque, B., Belgrano, A., Post, E., Reid, P. C. and Stenseth, N. C. 2001. Ecological effects of the North Atlantic Oscillation. Oecologia 128: 1-14. Schindler, D. W. 2001. The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millenium. Can. J. Fish. Aquat. Sci. 58: 18-29. Straile, D. and Adrian, R. 2000. The North Atlantic Oscillation and plankton dynamics in two European lakes – two variations on a general theme. Global Change Biology 6: 663-670. Straile, D. 2002. North Atlantic Oscillation synchronises plankton succession in Central European lakes. Proceedings of the Royal Society of London, B 269: 391-395. Straile, D., Livingstone, D. M., Weyhenmeyer, G. A. and George, D. G. 2003. The response of freshwater ecosystems to climate variability associated with the North Atlantic Oscillation. In: The North Atlantic Oscillation. Climate Significance and Environmental Impact. Eds.: J. W. Hurrell, Y. Kushnir, G. Ottersen and M. Visbeck. Geophysical Monograph Series 134: 263-279. |
|
 Vendance from ca. 5 to 15 cm (ages 0+ to 2-3+) caugth in Lake Vänern at the beginning of August 2001. Picture from Nyberg et al.6
Vendance from ca. 5 to 15 cm (ages 0+ to 2-3+) caugth in Lake Vänern at the beginning of August 2001. Picture from Nyberg et al.6 Photo: Lars Eriksson
Photo: Lars Eriksson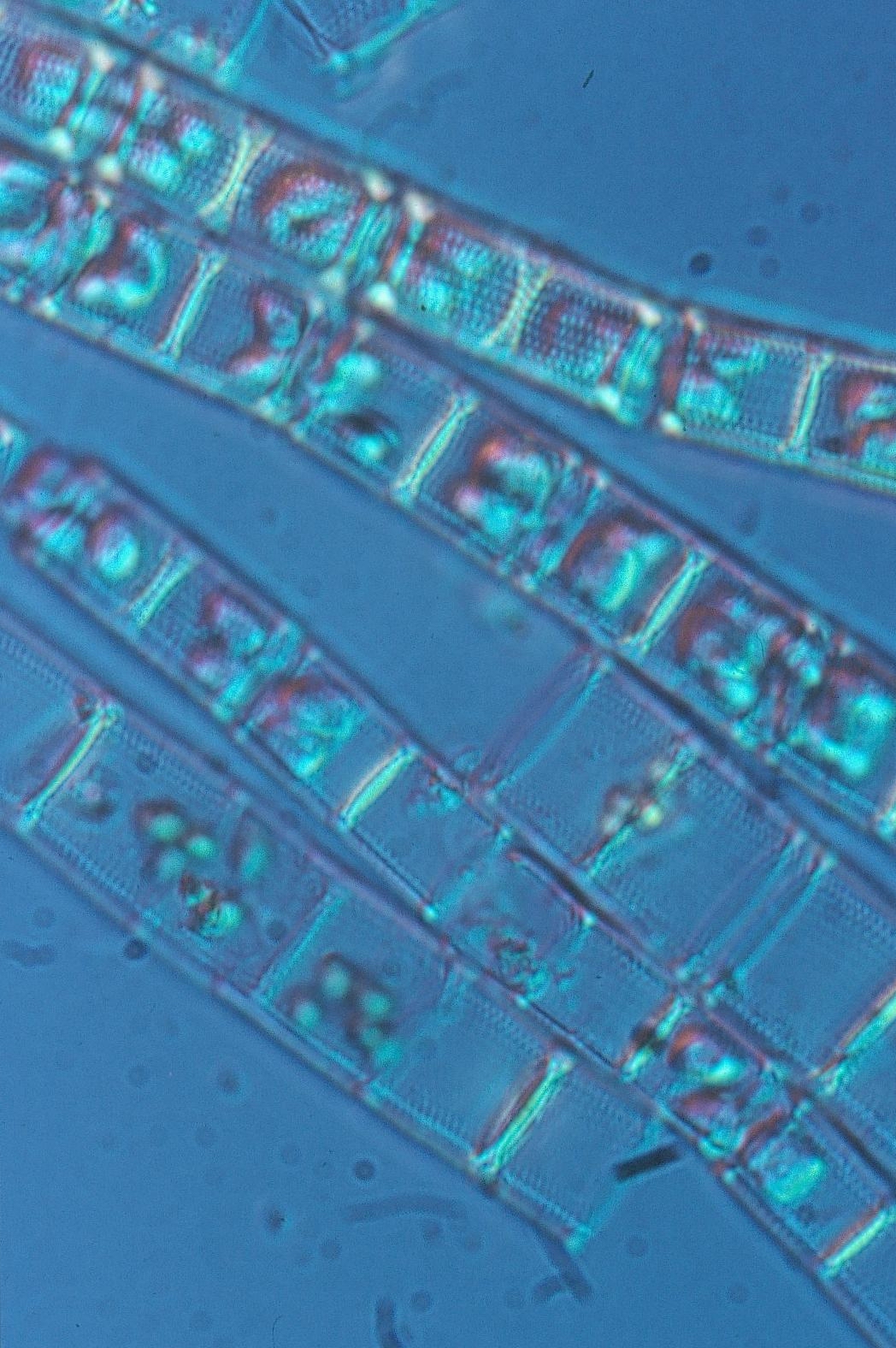 Photo: Eva Willén
Photo: Eva Willén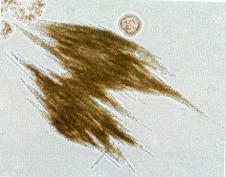 Photo: Eva Herlitz
Photo: Eva Herlitz